Figure 2.29
In Figure 2.29a the sodium chloride ion pair is selected as an example for strong electrostatic interactions between ions of opposite charge. In the accompanying text the strength of this interaction is described as:
"The gas-phase bond dissociation energy of this adduct for dissociation into the constituent ions amounts to BDE(NaCl) = +412 kJ mol-1, which puts this non-covalent interaction directly into the range of typical (covalent) C–H bond energies discussed before!"
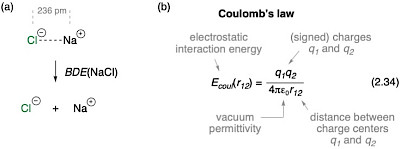
Fig. 2.29. (a) Dissociation of the sodium chloride ion pair into its constituent ions and (b) Coulomb's law.20,21
The BDE value cited here is, unfortunately and erronously, that of the ion pair dissociation to the respective radicals (the homolytic BDE), and not to the constituent ions (the heterolytic BDE illustrated in Fig. 2.29a)! This is carefully described by M. Vasiliu et al. in ref. 20, where the BDE(NaCl) value of 98.5 kcal mol-1 (412 kJ mol-1) is stated to refer to the homolytic dissociation to sodium and chlorine radicals as the reaction products. In order to arrive at the true reaction enthalpy for the ion pair formation reaction shown in Figure 2.29a, we have to additionally consider the energies for ionizing sodium to its cation (IP(Na) = 5.139076 eV) and adding an electron to chlorine (EA(Cl) = 3.612724 eV, both values taken from The Handbook of Chemistry and Physics, 89th edition, D. R. Lide (Ed.), 2008). Addition of the difference of 1.5264 eV (+147.27 kJ mol-1) to the homolytic BDE(NaCl) value cited before then yields the true heterolytic BDE(NaCl) = +559 kJ mol-1. The corrected accompanying text to Figure 2.29 then reads:
"The gas-phase bond dissociation energy of this adduct for dissociation into the constituent ions amounts to BDE(NaCl) = +559 kJ mol-1, which puts this non-covalent interaction directly into the range of typical (covalent) C–H bond energies discussed before!".
I am indepted to Dr. Armin Ofial (Dept. Chemistry, LMU Muenchen) for pointing me to this error.
Literature references for Figure 2.29:
20. M. Vasiliu, S. Li, K. A. Peterson, D. Feller, J. L. Gole and D. A. Dixon, J. Phys. Chem. A, 2010, 114, 4272.
21. Y.-R. Luo, Comprehensive Handbook of Chemical Bond Energies, CRC Press, 2007.